The importance of immune responses in breast carcinogenesis and treatment response
The intratumoral immunological landscape is increasingly being recognized as a major contributor to cancer development and progression. The concept of cancer immunosurveillance suggests that tumors are constantly eradicated by the immune system before clinical manifestations occur. However, leukocytes, which were once thought to be only implicated in cancer eradication, are now recognized for their dual functions in tumor promotion as well as inhibition.
In addition, cross-talks between the different immune cell types and the tumor cells are still poorly understood, as an example why T-cells from a melanoma patient recognize fewer neoantigens than the T cell of a healthy donor remains to be understood (Figure 1).
Given the increasing importance of immune constitution for the success of chemotherapy and targeted treatment, we study the cross-talks between the different immune cell types and the tumor cells.
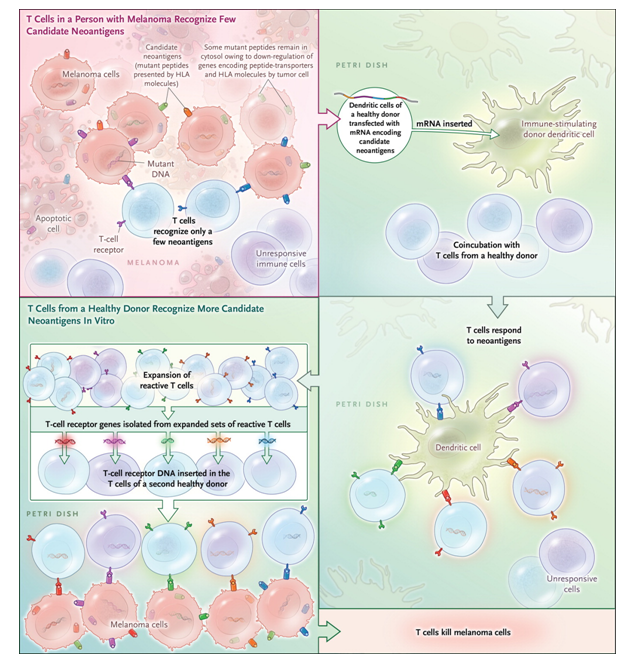
Figure 1. Outsourcing Cancer Immunity.Cancer cells harbor many mutations that produce potentially immunogenic mutant proteins that are, however, not recognized by the patient’s immune system. Strønen et al.4 recently reported a means of identifying donor-derived T-cell responses to such mutations. Using computer-based prediction algorithms, they first selected candidate neoantigens among peptides encoded by mutations from tumors of three patients with melanoma that — in theory — seemed likely to elicit an immune response. These mutant peptides were then synthesized and expressed in antigen-presenting (dendritic) cells obtained from a healthy donor and were coincubated with T cells from that same person. Neoantigen-reactive T cells activated by some of these transduced dendritic cells multiplied, and the “potent” T-cell receptor genes were isolated. T cells from a second healthy donor, when genetically engineered to express the neoantigen-reactive T-cell receptors, were shown to destroy tumor cells derived from the original melanomas. mRNA denotes messenger RNA. From The Antigenicity of the Tumor Cell - Context Matters. N Engl J Med. 2017 Feb 2;376(5):491-493. doi: 10.1056/NEJMcibr1613793. PMID:28146670
1. Immune cell infiltration relative to breast tumor subtypes
Our previous results demonstrated that the T-cell response and interleukin signaling pathways are important component to discriminate Her2 negative patients into groups with different prognosis.
Following studies showed that mutations in the tumor suppressor TP53 (mutated in approximately 30% of breast adenocarcinomas) could influence the lymphocytic infiltration in ER-negative breast tumors (Figure 2). These results underline the dependence between immune cells infiltration and tumor cells characteristics.
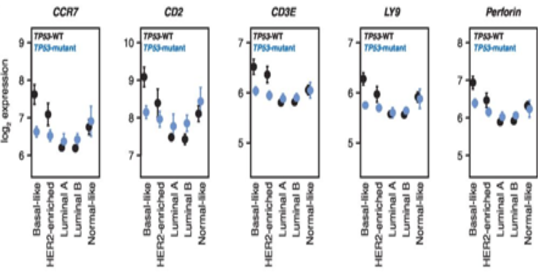
Figure 2. TP53 mutation status is associated with differential immumne cell infiltration in in Basal-like tumors.
Effect plots of expression of CCR7, CD2, CD3E, LY9, and perforin (PRF1) grouped by PAM50 subtype and TP53 mutation status. TP53-WT plotted in black, TP53-mutant plotted in blue. Points indicate mean, error bars indicate 95% confidence intervals calculated from twice the standard error. From Lymphocyte Invasion in IC10/Basal-Like Breast Tumors Is Associated with Wild-Type TP53. Mol Cancer Res. 2015 Mar;13(3):493-501. doi: 10.1158/1541-7786.MCR-14-0387. PMID:25351767
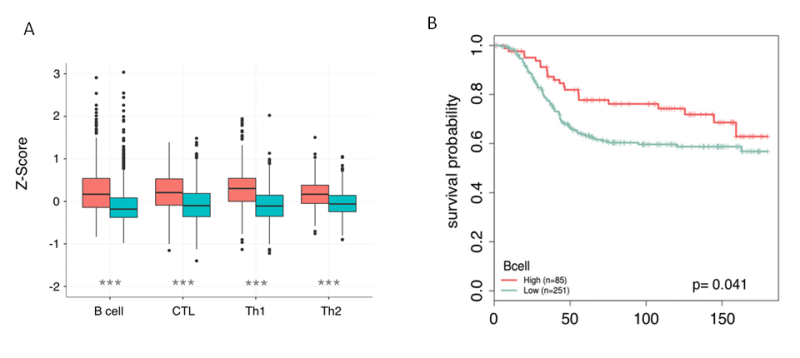
Figure 3. Nanodissection of breast cancer patients for Bcell, CTL, TH1 and Th2 infiltartion. A) Box plots show the increased levels of B cell, CTL, Th1, and Th2 infiltration in ER- samples when they are partitioned by estrogen receptor status. B) For each lymphocyte marker, patients are partitioned into high and low levels using quartiles. Patients with lymphocyte scores in the upper quartile are marked as high (red) and the remaining quartiles are grouped together and labeled low (blue). Increased lymphocyte infiltration is associated with better long term survival in ER- tumors(unpublished)
2. Non-invasive monitoring of immune activity in breast cancer patients
To elucidate mechanisms linking inflammation and cancer, we analyze cytokines levels in the plasma of breast cancer patients. We are comparing the levels of 27 cytokines in the plasma to the estimate immunological type observed in the core biopsies (Figure 4).This assessment of plasma levels of cytokines led us to discover that low levels of the chemokine IP10 is related to poor survival in breast cancer patients with luminal subtypes (Figure 5).
We also monitor the changes in cytokine levels in the plasma during and after treatment with targeted therapy Avastin.
The ultimate goal is to be able in the future by accurate measurement of the levels of a panel of cytokines in the plasma (non invasive methods) to estimate which immune cells invade the breast tumor and also predict patient response to treatments
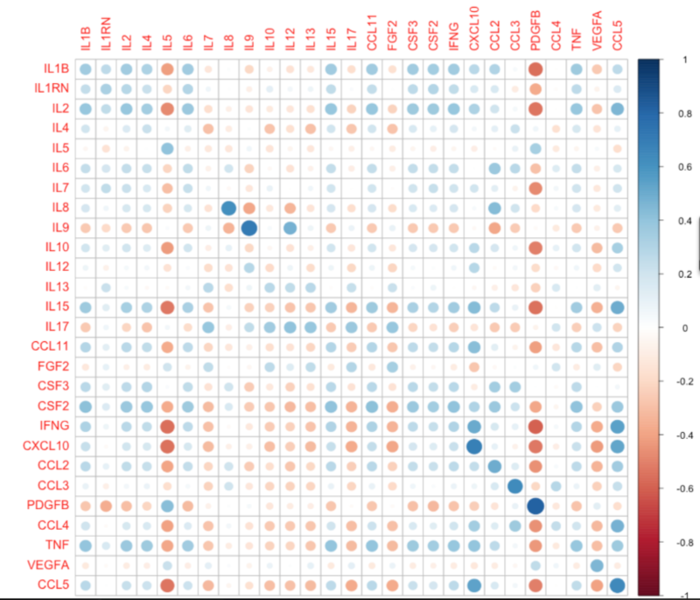
Figure 4. Comparison of cytokine levels in the plasma to their mRNA levels in the corresponding biopsied tissue (unpublished).
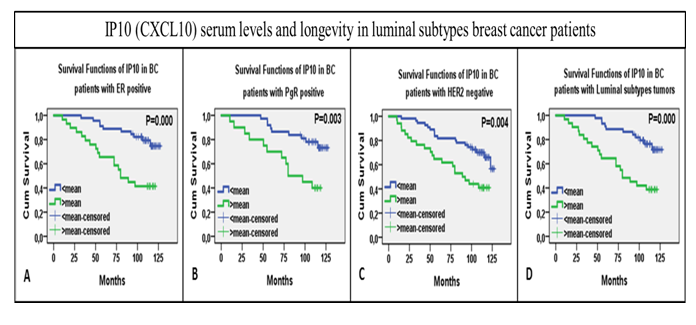
Figure 5. Plots for KM survival analysis with log rank test, KM survival curves shown over time on x-axis. (A) KM curves for overall IP10 serum levels distribution over time relative to cumulative survival. (B) KM curves for IP10 levels in ER positive tumor bearers (C) in PgR positive (C) in HER2 negative and (D) in luminal subtypes, A&B collectively (unpublished).
In total, our current and future research aims at using state-of-the-art in vitro (T cell Repertoire Sequencing, Nanostring…) techniques and in silico tools to accurately identify the immune cell types infiltrating tumors and their role in tumor pathogenesis. In parallel, we aim at developing non-invasive methods to characterize predict how the immune microenvironment affects breast cancer development.
Project members: Shakila Jabeen, Marianne Nome, Andliena Tahiri, Xavier Tekpli
External collaborators:
- Olga Troyanskaya http://function.princeton.edu/
- Irina Gromova, The Danish cancer Institute, Denmark https://www.cancer.dk/research/cell-death-metabolism/cdmsignaling/cdmsignalcoll/
- Alexandre Corthay, Rikshospitalet https://www.ous-research.no/corthay/
Recent publications:
1. The Antigenicity of the Tumor Cell - Context Matters.
N Engl J Med. 2017 Feb 2;376(5):491-493. doi: 10.1056/NEJMcibr1613793. PMID:28146670
2.Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling.
Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2802-7. doi: 10.1073/pnas.1108781108. PMID:21908711
3.Lymphocyte Invasion in IC10/Basal-Like Breast Tumors Is Associated with Wild-Type TP53.
Mol Cancer Res. 2015 Mar;13(3):493-501. doi: 10.1158/1541-7786.MCR-14-0387. PMID:25351767