Liquid biopsies in patient management
A urine-based biomarker test for detection and monitoring of bladder cancer
Aims
The specific aims of the present project are to:
- Identify accurate DNA methylation biomarkers for urine-based detection of bladder cancer
- Investigate the potential of the biomarkers to detect bladder cancer in two distinct clinical settings:
- Among patients with macroscopic hematuria1
- Among bladder cancer patients that are monitored for recurrence
- Demonstrate clinical utility and implement the biomarker urine test in routine clinical use
Highlights – the BladMetrix urine test
- High accuracy for detection and surveillance of bladder cancer across tumor grades and stages
- Potential to spare over half of the routine cystoscopies
- May contribute to patient-specific surveillance regimes through indications of early recurrences, minimal residual disease and field effect
- Intellectual property rights (IPR) are secured and commercial interest attracted
Background
Bladder cancer affects close to 600,000 people yearly and causes over 200,000 deaths [1]. The most common bladder cancer symptom is gross hematuria, which is present in almost 80% of newly diagnosed patients [2]. However, hematuria may also be caused by a variety of other genitourinary conditions, and the incidence of bladder cancer among hematuria patients is less than 20% [3]. About 70-75% of bladder cancer patients are diagnosed with non-muscle-invasive bladder cancer (NMIBC), and have up to 78% risk of recurrence after curative treatment [4, 5]. Moreover, around 20% with high risk disease will progress in stage [4]. The high recurrence and progression rates necessitate frequent patient surveillance.
The gold standard for both detection and surveillance of bladder cancer is cystoscopy. Cystoscopy is invasive, costly and has imperfect diagnostic accuracy, with only 71% sensitivity and 72% specificity reported in a meta-analysis [6]. Consequently, an accurate, cost-efficient and non-invasive test for detection of bladder cancer is warranted (Figure 1). A handful of urine tests have FDA-approval, and a variety of molecular biomarker tests have been suggested in the literature [7], but none are routinely used in the clinic.
Figure 1: A non-invasive, accurate and cost-efficient urine test for bladder cancer detection and surveillance that can replace or reduce the use of cystoscopy is warranted. Figure created with BioRender.com.
The BladMetrix urine test
By using methylome sequencing and an in-house established bioinformatics workflow, we have identified a bladder cancer specific biomarker panel, consisting of eight DNA methylation biomarkers. The biomarker panel has been developed into the BladMetrix test, and is analyzed by droplet digital PCR (ddPCR), using two in-house developed solutions. First, the 4Plex is a robust internal control assay included in each reaction that allows normalization of DNA input (Pharo et al., Clin Epigenetics, 2018 [8]). Second, the Positive Droplet Caller (PoDCall) performs automated calling of positive droplets through an R package and an accompanying shiny graphical user interface (Jeanmougin et al., Bioinformatics, 2023; [9]). These solutions ensure standardization and high accuracy of the analyses. An overview of biomarker discovery and clinical validation of BladMetrix is shown in Figure 2.
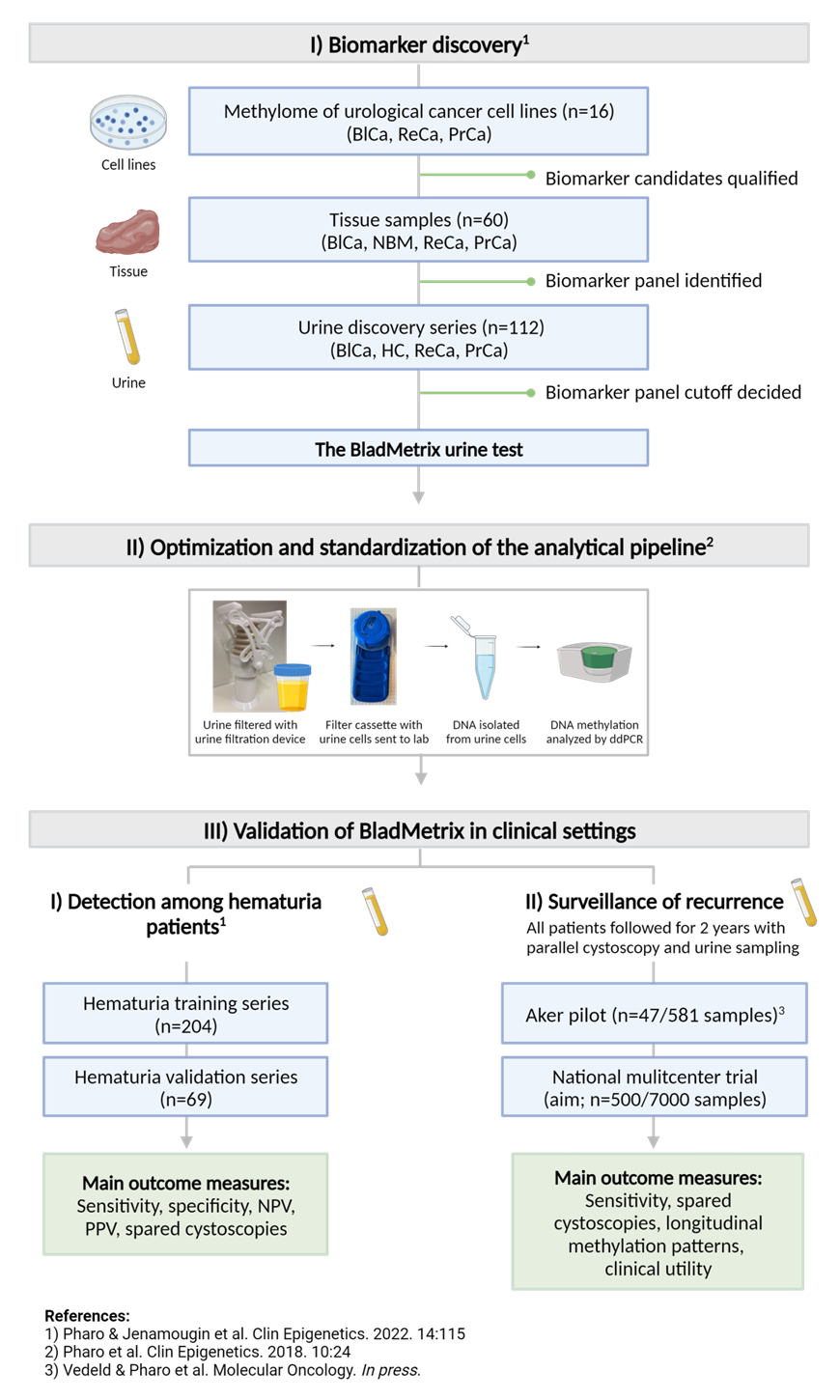
Figure 2: The overall project flow from discovery of biomarker candidates to clinical validation of the BladMetrix urine test. Abbreviations: BlCa Bladder cancer; NBM Normal bladder mucosa; HC Healthy controls; PrCa Prostate cancer; ReCa Renal cancer; NPV Negative predictive value; PPV Positive predictive value. Figure created with BioRender.com
Detection of bladder cancer among hematuria patients
In a large prospectively collected series of urine from patients presenting with gross hematuria (n=273) [10], BladMetrix achieved 92% sensitivity, 93% specificity and a negative predictive value (NPV) of 98% (Pharo et al., Clin Epigenetics, 2022; [11]). Importantly, the sensitivity remained high both among low-grade (85%) and high-grade (95%) tumors. With this accuracy level, BladMetrix outperforms both cystoscopy and other relevant competitor tests [12-14]. The study was performed together with our long-term collaborator Prof. Per Guldberg at the Danish Cancer Society.
Surveillance of bladder cancer recurrence – pilot study
In a pilot study, 47 patients with NMIBC were monitored for recurrence for two years, involving simultaneous cystoscopy controls and urine sampling, amounting to almost 300 data points. In this setting, BladMetrix identified 91% of the recurrences, including 83% of the Ta tumors, and showed potential to reduce the number of cystoscopies by 55% (Vedeld et al., Mol Oncol, 2024, in press). Moreover, distinct longitudinal patterns of urine tumor DNA (utDNA) suggested early recurrence detection, minimal residual disease and a methylation field effect, indicating that the BladMetrix test could potentially guide patient-specific surveillance regimes (Figure 3). This study was performed in collaboration with senior urologist and professor Rolf Wahlqvist and his team at Aker Hospital, Oslo University Hospital.
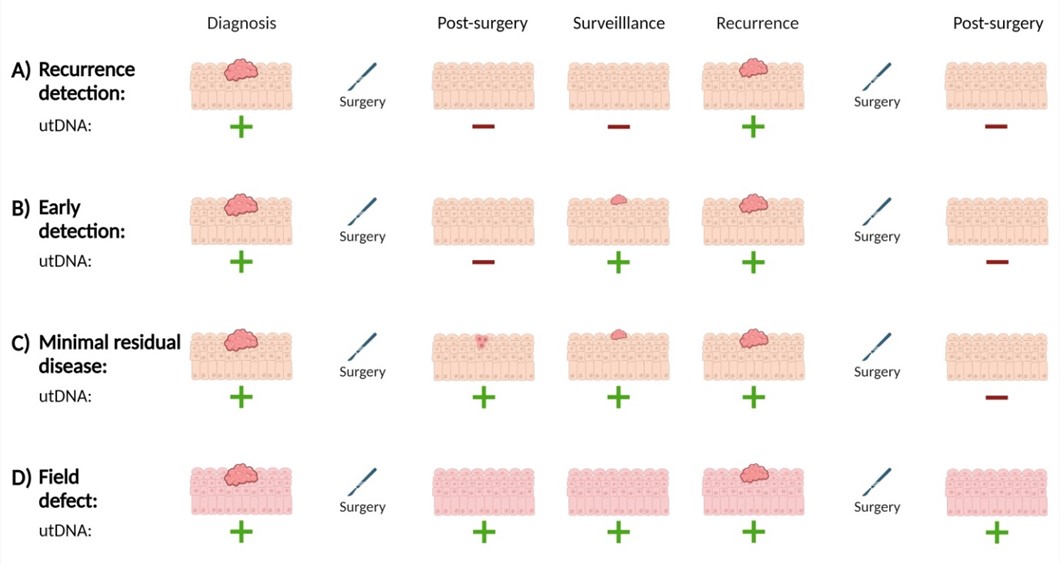
Figure 3: The BladMetrix test shows potential to identify various clinical scenarios through distinct longitudinal patterns of utDNA. Illustrative examples of utDNA patterns (positive: green plus sign, negative: red minus sign) at diagnosis, post-surgery, during surveillance and recurrence in the setting of A) regular recurrence detection, B) early detection, C) MRD; and D) field effect. Abbreviations: MRD, minimal residual disease; utDNA, urine tumor DNA. Figure created with BioRender.
Surveillance of bladder cancer recurrence- a clinical multicenter trial
In order to validate the promising results from the surveillance pilot study and demonstrate clinical utility, we are currently performing a large national multicenter trial. By December 2023, over 600 patients had been enrolled, and all patients will be followed for 2 years with urine sampling at every cystoscopy control. It is expected that around 7000 urine samples will be biobanked. The results are expected by 2026, and will hopefully prove clinical utility for the BladMetrix test, followed by implementation in the clinic, initially in Norway. Six hospitals from all health regions in Norway are participating in this study, including Aker Hospital, Akershus University Hospital, Vestfold Hospital Trust, Haukeland University Hospital, St. Olavs Hospital and The University Hospital of North Norway. Prof. Guro E. Lind is the principal investigator (PI) and Prof. Rolf Wahlqvist the head clinician. Discussions for licensing of the test to a European biotech company are ongoing.
Innovation and funding
The BladMetrix test is protected by to patent applications. One granted US (US9797016) and EP (2,630,261) and one pending (WO/2020/099938). BladMetrix has also attracted commercial interest.
This work has been supported by grants from the South‑Eastern Norway Regional Health Authority (project number 40093 and project number 2019074), the Research Council of Norway (project number 300741/H10) and KLINBEFORSK (project number 2018203), as well as a Cancer‑BIOTEK Grant No (254834), and innovation funding from the South‑Eastern Norway Regional Health Authority and the University of Oslo, Norway.
Acknowledgements
We are very thankful to our patient user board consisting of former and current bladder cancer patients, led by Elin Schive, providing valuable feedback and input from a patient perspective.
Patient summary
Cystoscopy is the gold standard for both detection and surveillance of bladder cancers, but is invasive and uncomfortable. We have developed the urine test “BladMetrix” that can detect bladder cancer with high accuracy. Among patients with visible blood in the urine (hematuria), BladMetrix detects 92% of the bladder cancer cases. Among patients monitored for recurrence, BladMetrix detects 91% of the recurrences. Altogether, our data indicate that use of BladMetrix in the clinic could have spared over 50% of the currently performed routine cystoscopies.
1These patients qualify for a standardized “fast track” schedule for cancer investigation in Norway; “Pakkeforløpet for kreft”: https://www.helsedirektoratet.no/nasjonale-forlop/blaerekreft.
References
[1] GLOBOCAN. Global Cancer Statistics 2020 [Online]. Available: http://gco.iarc.fr/
[2] D. Ramirez, A. Gupta, D. Canter, B. Harrow, R. W. Dobbs, V. Kucherov, et al., "Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer," BJU Int, vol. 117, pp. 783-6, May 2016.
[3] B. P. Rai, J. Luis Dominguez Escrig, L. Vale, T. Kuusk, O. Capoun, V. Soukup, et al., "Systematic Review of the Incidence of and Risk Factors for Urothelial Cancers and Renal Cell Carcinoma Among Patients with Haematuria," Eur Urol, vol. 82, pp. 182-192, Aug 2022.
[4] A. T. Lenis, P. M. Lec, K. Chamie, and M. D. Mshs, "Bladder Cancer: A Review," Jama, vol. 324, pp. 1980-1991, Nov 17 2020.
[5] S. van den Bosch and J. Alfred Witjes, "Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review," Eur Urol, vol. 60, pp. 493-500, Sep 2011.
[6] G. Mowatt, J. N'Dow, L. Vale, G. Nabi, C. Boachie, J. A. Cook, et al., "Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis," Int J Technol Assess Health Care, vol. 27, pp. 3-10, Jan 2011.
[7] E. Laukhtina, S. R. Shim, K. Mori, D. D'Andrea, F. Soria, P. Rajwa, et al., "Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis," Eur Urol Oncol, Nov 6 2021.
[8] H. D. Pharo, K. Andresen, K. C. G. Berg, R. A. Lothe, M. Jeanmougin, and G. E. Lind, "A robust internal control for high-precision DNA methylation analyses by droplet digital PCR," Clin Epigenetics, vol. 10, p. 24, 2018.
[9] M. Jeanmougin, H. P. Brodal, H. Dietrichson Pharo, H. M. Vedeld, and G. E. Lind, "PoDCall: positive droplet calling and normalization of droplet digital PCR DNA methylation data," Bioinformatics, vol. 39, Jan 1 2023.
[10] C. M. Dahmcke, K. E. Steven, L. K. Larsen, A. L. Poulsen, A. Abdul-Al, C. Dahl, et al., "A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria," Eur Urol, vol. 70, pp. 916-919, Dec 2016.
[11] H. D. Pharo, M. Jeanmougin, E. Ager-Wick, H. M. Vedeld, A. K. Sørbø, C. Dahl, et al., "BladMetrix: a novel urine DNA methylation test with high accuracy for detection of bladder cancer in hematuria patients," Clin Epigenetics, vol. 14, p. 115, Sep 17 2022.
[12] P. O'Sullivan, K. Sharples, M. Dalphin, P. Davidson, P. Gilling, L. Cambridge, et al., "A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria," J Urol, vol. 188, pp. 741-7, Sep 2012.
[13] K. E. van Kessel, W. Beukers, I. Lurkin, A. Ziel-van der Made, K. A. van der Keur, J. L. Boormans, et al., "Validation of a DNA Methylation-Mutation Urine Assay to Select Patients with Hematuria for Cystoscopy," J Urol, vol. 197, pp. 590-595, Mar 2017.
[14] E. Wallace, R. Higuchi, M. Satya, L. McCann, M. L. Y. Sin, J. A. Bridge, et al., "Development of a 90-Minute Integrated Noninvasive Urinary Assay for Bladder Cancer Detection," J Urol, vol. 199, pp. 655-662, 2018.